|
 |

 |
|
|
THE PHYSICS
OF VACUUM POTS
The operation of a vacuum coffee pot is a
source of considerable fascination, but the physical processes involved are often not
clearly understood. The following is an attempt to demystify the physics of the vacuum
brewing process.


T = 294 K
Va = 0.237 liters

|
Step 1:
When the vacuum pot is
assembled, we have an essentially closed (air-tight) lower chamber which contains water
and, in the space above the water, a mixture of air and water vapor. The
concentration of water vapor depends only on the temperature. It is not dependent on
the quantity of water and is only slightly influenced by the presence of the air in the
chamber. |
| The total pressure
(Pt) inside the lower chamber is a combination of the pressure exerted by the air (air
pressure) and by the water vapor (vapor pressure). |
| The air pressure
(Pa) exerted on the walls of the container and on the surface of the water is expressed by
the Ideal Gas Law, which approximates the behavior of ideal gases at low pressures: |
| Pa
= nRT / V |
where
Pa is air pressure (atm)
V is Volume (liters)
n is quantity of gas (moles)
R is the universal gas constant
T is temperature (K) |
| If we
assume a starting condition of the vacuum pot with its lower chamber filled with water to
seven-eighths capacity, and an air pressure (Pa) at room temperature (294K) of 1 atm, we
can estimate the quantity of air inside the lower chamber: |
PV = nRT
n = PaVa/RT
n = (1 atm) (0.237 liters) / (0.0821 liter-atm/mole-K) (294 K)
n = 0.00982 moles |
| The
vapor pressure (Pv) at the starting point is essentially zero. |
| Pv
= 0 atm |
|
| The
total pressure in the lower chamber is the sum of the air pressure (Pa) and the vapor
pressure (Pv). At room temperature (294K), this yields the following: |
Pa = 1 atm
Pv = 0 atm
Pt = Pa + Pv = 1 atm + 0 atm = 1 atm |
|
|
T = 363 K
Va = 0.828 liters
|
Step 2:
As heat is added to the system,
several changes begin to occur. In the lower chamber, a saturated condition develops
and the vapor pressure (Pv) exerted on the walls of the container and on the surface of
the water increases exponentially with temperature. The saturated vapor pressure can
be approximated by the equation [F. W. Murray, 1966]
: |
| Pv = 0.006 · exp[ a · (T - 273.16) / (T - b)] |
where
Pv is vapor pressure (atm)
a is the constant 17.27 over water
b is the constant 35.86 over water
T is temperature (K) |
| At about 353K, air
dissolved in the water starts to be released as tiny bubbles and the air and water vapor
over the surface of the water increase in temperature. When the total pressure (Pt)
exceeds the external air pressure (1 atm), the force on the surface of the water inside
the lower chamber is greater than the force on the surface of the water in the funnel tube
and upper chamber. As a result, water from the lower chamber is gradually forced up the
funnel tube into the upper chamber where it mixes with the ground coffee. The greater the
total pressure, the higher the water rises. [Note that we have chosen to ignore any
resistance from the ground coffee or filtering mechanism.] |
| If we use the
temperature of the water half-way down the funnel tube to approximate the temperature of
the whole system, we can calculate the total pressure (Pt) in the chamber as a function of
temperature. Experimental measurements of the water temperature (T) and
corresponding gas volume (V) in the chamber yield the following: |
| T (K) |
V (l) |
| 298 |
0.237 |
| 303 |
0.242 |
| 308 |
0.247 |
| 313 |
0.248 |
|
| T (K) |
V (l) |
| 318 |
0.266 |
| 323 |
0.274 |
| 328 |
0.286 |
| 333 |
0.300 |
|
| T (K) |
V (l) |
| 338 |
0.307 |
| 343 |
0.319 |
| 348 |
0.367 |
| 353 |
0.414 |
|
| T (K) |
V (l) |
| 358 |
0.532 |
| 363 |
0.828 |
| 368 |
1.064 |
| 373 |
1.419 |
|
| Remembering that
the total pressure (Pt) is the sum of the air pressure (Pa) and vapor pressure (Pv), we
can now estimate the total pressure at any point in the process after saturation has
occurred. For example, at 363K: |
Pa = nRT/Va
Pa = (0.0107 moles)(0.0821 liter-atm/mole-K)(363 K) / (0.828 liters) = 0.389 atm
Pv = 0.006 · exp[ a · (T - 273.16) / (T - b)]
Pv = 0.006 · exp[ 17.27 ·
(363 - 273.16) / (363 - 35.86)] = 0.698 atm
Pt = Pa + Pv = 0.389 atm + 0.698 atm = 1.087 atm |
| Throughout this process, the
system remains in dynamic equilibrium, with increasingly more liquid water escaping as
vapor (due to the increase in kinetic energy resulting from the increase in temperature)
and vapor condensing back into liquid water at an equivalent rate. |
|
|

T = 368 K
Va = 1.064 liters
|
Step 3:
As the total pressure (Pt)
continues to increase, the volume of water in the lower chamber decreases and the volume
of gas increases until the water's surface reaches the bottom of the funnel tube.
The temperature measured experimentally at this stage was 368K. |
| Up to this point, the
quantity of water vapor and the associated vapor pressure in the chamber has steadily
increased, but the quantity of air molecules has remained essentially constant (it's an
air-tight system). Remembering that the quantity of air calculated in Step 1 was
0.0097 moles, and allowing for an estimated 10%** increase in air molecules due to the
release of air dissolved in the water (0.0097 moles x 1.10 = 0.0107 moles), we can
estimate the air pressure (Pa) at 368K: |
PV = nRT
Pa = nRT/Va
Pa = (0.0107 moles)(0.0821 liter-atm/mole-K)(368 K) / (1.064 liters)
Pa = 0.304 atm |
| The air pressure (Pa) in the
system has actually decreased to about one third it's starting value, and therefore can
not be the dominant force in the vacuum brewing process. The increase in volume in
the lower chamber has effectively canceled out any pressure gains produced by the
increased temperature. |
| Calculating vapor pressure at
this point reveals the greater force behind the brewing process: |
Pv
= 0.006 · exp[ a · (T - 273.16) / (T - b)]
Pv = 0.006 · exp[17.27 · (368 - 273.16) / (368 -35.86)] atm
Pv = 0.842 atm |
| Again, the total pressure
(Pt) is the combination of the two: |
Pt = Pa
+ Pv
Pt = 0.304 atm + 0.842 atm
Pt = 1.146 atm |
| This pressure exceeds the external pressure
(1 atm) and is sufficient to force the water up the funnel tube. |
| ** This
estimate is based on the combined solubility of the three primary constituents of air in
water at 1 atm, weighted according to their relative percentages: Nitrogen (79%), Oxygen
(20%) and Argon (1%). |
|
|

T = 373 K
Va = 1.419 liters
|
Step 4:
As we continue to heat the
system, the total pressure (Pt) forces the surface of the water in the lower chamber
below the bottom of the funnel tube. At this point the system is no longer closed.
The pressurized gas in the lower chamber is allowed to escape and it bubbles up through
the liquid in the upper chamber. The escaping gases include both air and water
vapor, resulting in a net decrease in the quantity of air in the lower
chamber. |
| We can calculate the
theoretical maximum total pressure (Pa) at Step 4: |
Pa = (0.0107
moles)(0.0821 liter-atm/mole-K)(373 K) / (1.419 liters) = 0.231 atm
Pv = 0.006 · exp[17.27 · (373 - 273.16) / (373 -35.86)] atm = 1 atm
Pt = 0.231 atm + 1 atm
Pt = 1.231 atm |
| At this point in the process,
the water in the lower chamber begins to boil. Large bubbles of water vapor rise and
break the surface of the water. In the normal
brewing process, the heat is lowered at this point and the whole system is allowed to
"gurgle" for several minutes. This further reduces the amount of air in
the lower chamber as air molecules are pushed out and replaced by water vapor. The
amount of air removed during this process depends on the length of time that the system is
allowed to "gurgle". By estimating the bubble size and escape rate, and
assuming good mixing of the gases and an exponential decay of air (as a percentage of the
total volume of gases) over time, we can calculate the amount of air remaining in the
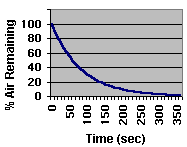
lower chamber as a function of "gurgle" time. |
| Gurgle
Time |
Percent
of Air Remaining |
| 1 minute |
49% |
| 2 minutes |
25% |
| 3 minutes |
12% |
| 4 minutes |
6% |
| 5 minutes |
3% |
|
|
|
| In order for a sufficient vacuum to form to
complete the brewing process, it appears that the ratio of air to water vapor achieved
during the "gurgling" process must reach a specific value. The quantity of
air molecules at the starting point varies directly with the initial water level -- the
less water used, the more air in the chamber. As a result, a longer or shorter
"gurgle" time is required to reach the critical air/vapor ratio, depending on
the starting water level. Experimental results show the maximum allowable quantity
of post-gurgle air molecules to be approximately 0.005 moles. This allows us to suggest
minimum gurgle times for different initial water levels for an 8-cup vacuum pot: |
Starting
Water
Level |
Air
Molecules
(initial) |
Air
to Vapor Reduction Req'd |
Minimum
Estimated Gurgle Time |
| 7/8 full |
0.00982 moles |
49% |
1 minute |
| 2/3 full |
0.0196 moles |
74% |
2 minutes |
| 1/2 full |
0.343 moles |
85% |
3 minutes |
|
| Click here for
comparative plots of Pressure as a function of "gurgle" time. |
| It should be remembered that the force of the
vacuum must also overcome any resistance caused by the ground coffee and filter mechanism.
Since this varies so widely with the fineness of the grind and the design of the fiter, we
are forced to ignore this factor in our calculations. |
| In this model, we will assume a two minute
gurgle time and a corresponding 75 percent reduction in air molecules during the time span
that the system remains open such that Va (final) = 0.25 Va (initial). |
| Note: The increase in
pressure inside the lower chamber elevates the boiling point of the water by about 1
degree K for each 28 mmHg (0.0037 atm), which in our case would result in a theoretical
boiling point of about 378K. But since boiling generally occurs only after the system has
become open (at which point the actual pressure in the lower chamber is difficult to
determine), this factor has been ignored in this model. |
|
|

T < 373 K
Va < 1.419 liters
|
Step 5:
After two minutes of
"gurgling", the heat is removed from the system and the gases in the lower
chamber begin to cool. As a result, the volume of air decreases and the water vapor
begins to condense to liquid more quickly than the water evaporates to vapor.
Consequently, the total internal pressure decreases. When the internal pressure
falls below the external pressure (1 atm), the water in the upper chamber is forced back
down the funnel tube into the lower chamber. |
| We can calculate the
approximate temperature at which the downward flow begins. |
Pt = Pa
+ Pv = 1 atm
Pa = nRT/Va
Pv = 0.006 · exp[ a · (T - 273.16) / (T - b)]
nRT/Va + 0.006 · exp[
a · (T - 273.16) / (T - b)] = 1 atm
solving for T, we find:
T = 369 K |
| As soon as the water level in the lower
chamber rises above the bottom of the funnel tube, the system becomes closed once
again. As the temperature continues to drop, the water vapor condenses rapidly,
causing the total internal pressure (Pt) to drop precipitously. The difference
between the internal and external pressures results in the formation of a partial vacuum
within the lower chamber, and the higher external pressure forces the brewed coffee
rapidly into the lower chamber. |
|
|

T = 353 K
Va = 0.237 liters
|
Step 6:
When all the brewed coffee has
been pushed down into the lower chamber there may still be sufficient vacuum in the lower
chamber to force a quantity of air down the funnel tube as well, with air bubbles visibly
rising from the bottom of the funnel. |
| We can calculate the
approximate total pressure just as the coffee has completely returned to the lower
chamber: |
Pa = (0.0107
moles)(0.25)(0.0821 liter-atm/mole-K)(353 K) / (0.237 liters)
Pv = Pv = 0.006 · exp[17.27 · (353 - 273.16) / (353 -35.86)] atm
Pt = Pa + Pv
Pt = 0.327 atm + 0.470 atm = 0.797
atm |
| At this point, the pressure
in the lower chamber is still substantially lower than the external air pressure of 1 atm
as predicted. The bubbling of air partially equalizes the internal and external pressures,
but can not succeed completely due to the work required to push the air bubbles down the
tube. |
|
|

T = 348 K
Va = 0.237 liters
|
Step 7:
At the end of the process, all
the coffee has returned to the lower chamber, and some of the difference between the
internal and external pressures has been equalized. The process ends with a small
but perceptible vacuum in the lower chamber, requiring a slight effort to remove the upper
chamber. |

To review, it is the combination of
air pressure and vapor pressure that work together to push the water upward in the first
stage of the vacuum brewing process, but it is primarily the condensation or phase change
of the water vapor which creates the partial vacuum, and thereby triggers the second
stage.



 |
|
|
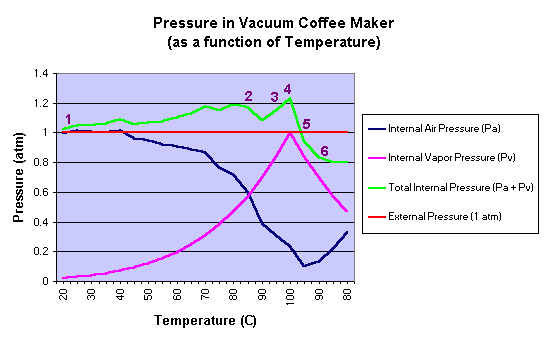
Process Overview:
It may be interesting to
inspect a plot of the pressures involved in the vacuum brewing process as a function of
temperature. The plot shown below is based upon a model developed from the equations
and approximations described above. |
|
Rubberless
Vacuum Brewers:
Brewing coffee in one of the
"rubberless" (i.e. gasket-less) vacuum brewers poses special consideration to
one wishing to understand the physics of the vacuum coffee pot. In these brewers
there is no rubber gasket to form an air-tight seal between the upper and lower chambers.
Instead, the combined weights of the upper chamber, filter, and ground coffee must
be sufficient to counter the total internal pressure exerted upward which would tend to
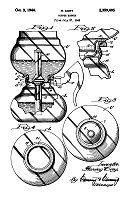
dislodge the upper chamber. Here is how the inventor, Harvey Cory, explained it: |
| "The seal
provided by the ground joint is maintained fluid tight by the weight of the upper bowl
plus the rod. The pressure developed during the brewing operation tends to lift the
upper bowl and break the seal. This pressure is determined by the height the water
must rise before its weight in the upper bowl more than compensates for the additional
difference in levels. In the case of an upper bowl of eight-cup capacity (about 46
ounces), its weight, plus that of the rod, may be about 14 to 15 ounces, and with the
ground coffee therein, the total weight is about .95 pound. The height to which the
water must rise depends upon the amount thereof which is displaced from the lower bowl.
Under ordinary conditions this may require a head of from two and a half to three
and a half inches. If the mean diameter of the sealing surfaces is X inches, then
the value X for the pressure conditions will have to be balanced will be as follows: |
 |
|

|
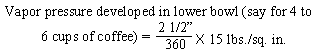
|
For balanced condition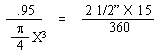
|
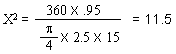
|

|
| "This figure of 3.4 inches diameter which is
theoretical requires that some factor of safety be allowed. For instance, it is
found that with the rod and ground coffee in place, some restriction is offered to the
up-flow of water into the upper bowl. This will increase the effective internal
pressure required to raise the water into the upper bowl. There is also the weight
pressure which must overbalance the internal pressure by a small amount in order that the
seal may remain fluid tight. For these reasons, two and seven-eighths inches
represents a practical maximum value for the X dimension in the case of a coffee maker of
the size indicated." [Excerpt
from U.S. Patent No. 2359405, dated Oct. 3, 1944] |
|
|
|

Copyright © 2001-2024 BHA
Enterprises
All Rights Reserved
Address comments to: Brian Harris |

|
|
|